
Celebrating UW Medicine Faculty's Innovative Contributions to Healthcare
The UW Medicine Inventor of the Year award honors outstanding UW scientists whose inventions have had a major effect on both human health and our local economy. Each year, the Inventor of the Year selection committee solicits nominations from department chairs and administrators which are then reviewed based on the following criteria:
- Number of lives saved or improved
- Biomedical impact of the invention
- Contribution to the Bioscience sector
- Contributions to the UW CoMotion mission to extend the impact of University of Washington research through the creation of partnerships that encourage investment in innovation.
- Contributions to the UW School of Medicine faculty community.
Current and Former Award Recipients
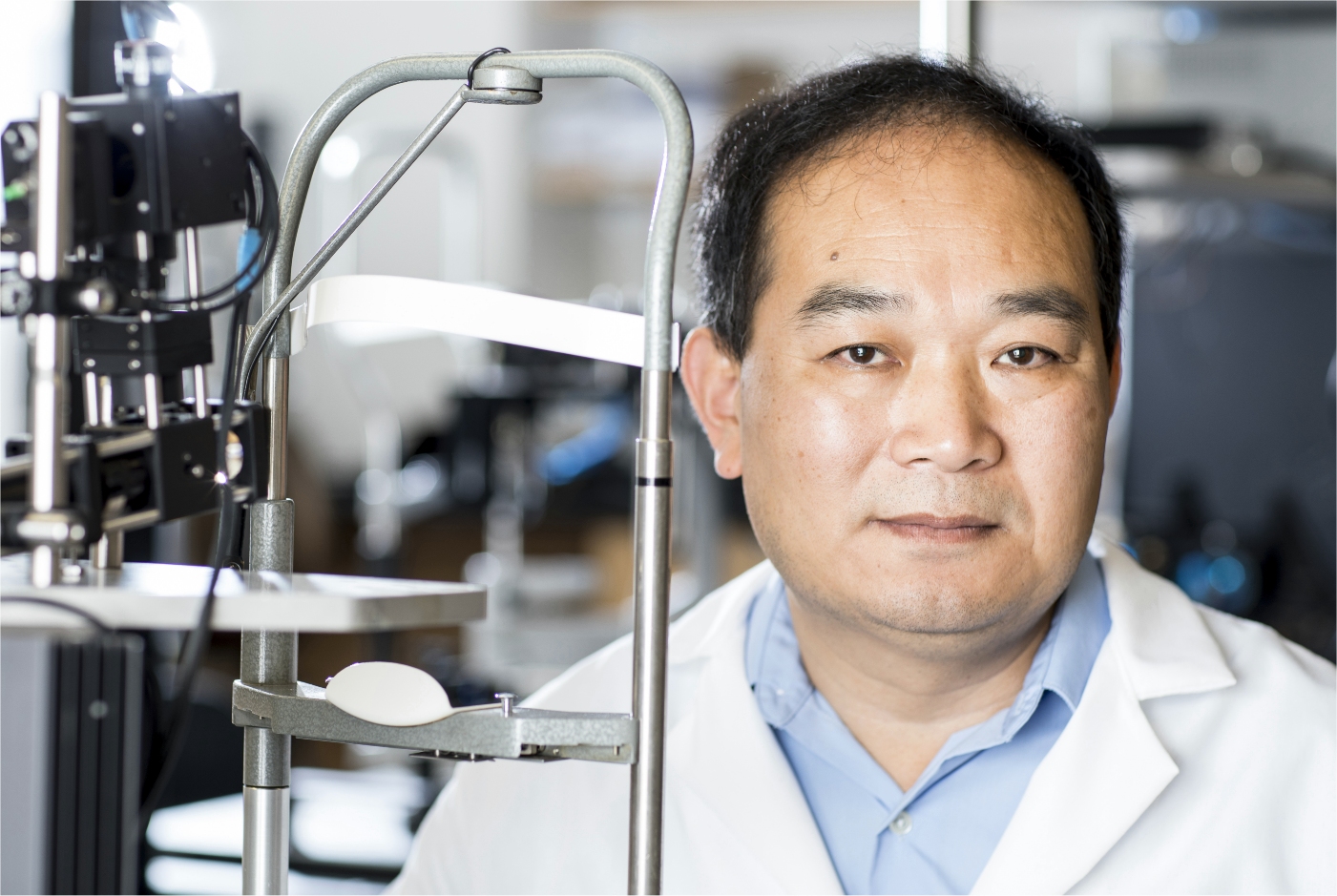
Pictured: Ruikang “Ricky” Wang, PhD
2023 Inventor of the Year - Ruikang "Ricky" Wang, PhD
Dr. Wang is currently a professor of Bioengineering and Ophthalmology and holds George and Martina Kren Endowed Chair in Ophthalmology Research at the University of Washington. His laboratory is responsible for the invention of optical coherence tomography angiography (OCTA, or optical microangiography), an OCT imaging technique capable of 3D imaging of blood flow within microcirculatory tissue beds in vivo. He has published extensively on this topic, including demonstrations of the use of OCTA to image retinal blood flow, cerebral cortical blood flow, cochlear blood flow, skin blood flow and oral cavity, without a need for contrasting dyes. The technique has now been translated for use in clinical ophthalmology, making a real impact on saving patient vision. His laboratory has also made important contributions to the current development of cellphone-based smart healthcare, where they invented a cost-to-nothing technique to realize hyperspectral imaging on unmodified smartphones. This innovation will enable widespread use by general public because of the increasingly popular use of smartphones in our daily life.
In the News
Ruikang Wang is 2023 UW Medicine Inventor of the Year
Ricky Wang, PhD to be honored as UW Inventor of the Year Nov. 1
UW Medicine Inventor of the Year: Ruikang Wang

2022: Jay Shendure, MD, PhD
Dr. Shendure’s research group in Seattle pioneered exome sequencing and its earliest applications to gene discovery for Mendelian disorders and autism; cell-free DNA diagnostics for cancer and reproductive medicine; massively parallel reporter assays, saturation genome editing; whole organism lineage tracing, and massively parallel molecular profiling of single cells.

2020: Alexander Greninger, MD, PhD, MS, M.Phil., and Keith Jerome, MD, PhD
The 2020 Inventor of the Year Award honored Alexander Greninger, MD, PhD, MS, M.Phil., and Keith Jerome, MD, PhD for their COVID-19 polymerase chain reaction (PCR testing) design.

2019: Jim Stout, PhD
Dr. Stout recognized the need for improved training and set about to create computer-based instruction to improve spirometry testing and the quality and accuracy of results. Subsequently re-developed as a web-based suite of five separate training programs, the Spirometry 360® resources have been licensed to individual healthcare practices, hospital systems, US states, and also to international healthcare services and brought over $2M in licensing revenue into UW.
Past Award Winners
2018: Thomas S Lendvay, MD
2017: Christy McKinney, PhD, MPH & Michael Cunningham, MD, PhD
2016: Samuel Browd, MD, PhD., Jonathan Posner, PhD
2015: David R. Eyre, PhD
2014: David Russell, MD, PhD
2013: Fred Silverstein, MD
2012: Yongmin Kim, PhD
2011: David Baker, PhD
2010: Roy Martin, PhD
2009: Bonnie Ramsey, MD, Arnold Smith, MD, Bruce Montgomery, MD
2008: Irwin D. Bernstein, MD
2007: Phillip Green II, PhD
2006: David C. Auth, PhD
2005: Earle W. Davie, PhD
2004: Benjamin Hall, PhD
